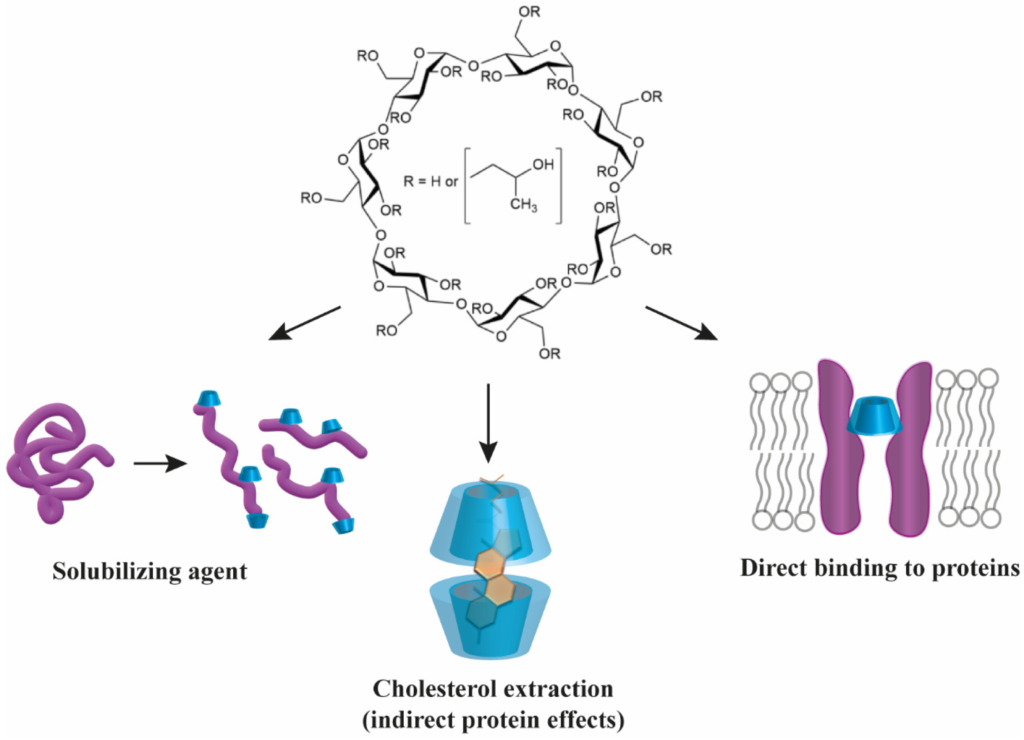
Even though used in many formulations with CDs, new ones entering clinical trials are not often. Midatech Pharma PLC recently announced recruiting the first patient in the Phase 1 Study of MTX-110 for recurrent #glioblastoma (NCT 05324501). Midatech has previously reported encouraging results from a Phase I study of MTX110 in diffuse intrinsic pontine glioma (NCT03566199, NCT04264143), and a Phase I study of MTX110 in medulloblastoma is being undertaken. and recurrent medulloblastoma (NCT04315064).
MTX110 is a water-soluble form of a panobinostat-free base, achieved through complexation with hydroxypropyl-β-cyclodextrin (HPBCD), that enables convection-enhanced delivery (CED) at potentially chemotherapeutic doses directly to the site of the tumor. Panobinostat is a hydroxamic acid and acts as a non-selective histone deacetylase inhibitor (pan-HDAC inhibitor). The currently available oral formulation of panobinostat lactate (Farydak®) is not suitable for the treatment of brain cancers owing to poor blood-brain barrier penetration and inadequate brain drug concentrations.
MTX110 is delivered directly into and around the patient’s tumor via a catheter system (e.g., CED or fourth ventricle infusions) to bypass the blood-brain barrier. This technique exposes the tumor to very high drug concentrations while simultaneously minimizing systemic drug levels and the potential for toxicity and other side effects.
MTX110 has been granted Orphan Drug and Fast Track designations by the FDA and Orphan Medicinal Product designation by EMA.
See the full article here