Insight into oral amphiphilic cyclodextrin nanoparticles for colorectal cancer: comprehensive mathematical model of drug release kinetic studies and antitumoral efficacy in 3D spheroid colon tumors
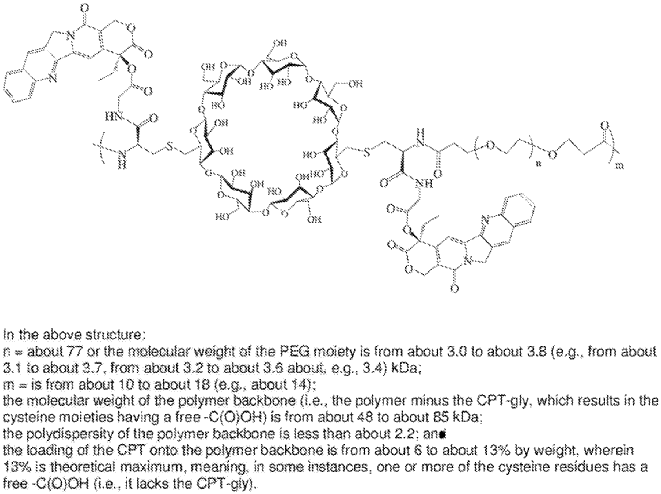
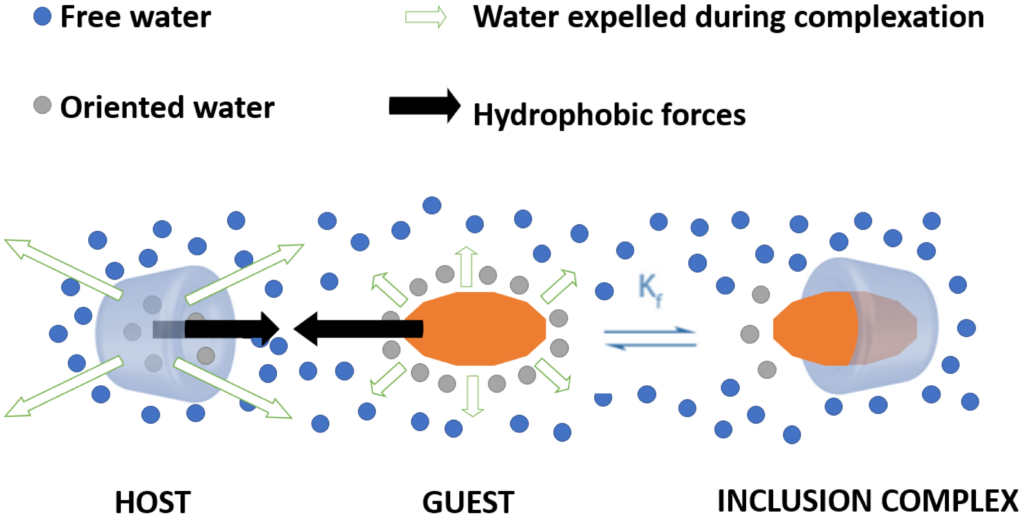
It is fascinating how similar concepts can be used for entirely different purposes in drug delivery: while we use amphiphilic cyclodextrin for packaging oligonucleotides in the GENEGUT project, Erem Bilensoy and her team at Hacettepe University created camptothecin-loaded nanoparticles to treat colon cancer. The overall findings indicated that the strategy of orally targeting anticancer drugs such as CPT with positively charged poly-β-CD-C6 nanoparticles to colon tumors for local and/or systemic efficacy is promising.
Dr. SEDAT ÜNAL (PharmD/PhD), Gamze Varan, Yeşim Aktaş