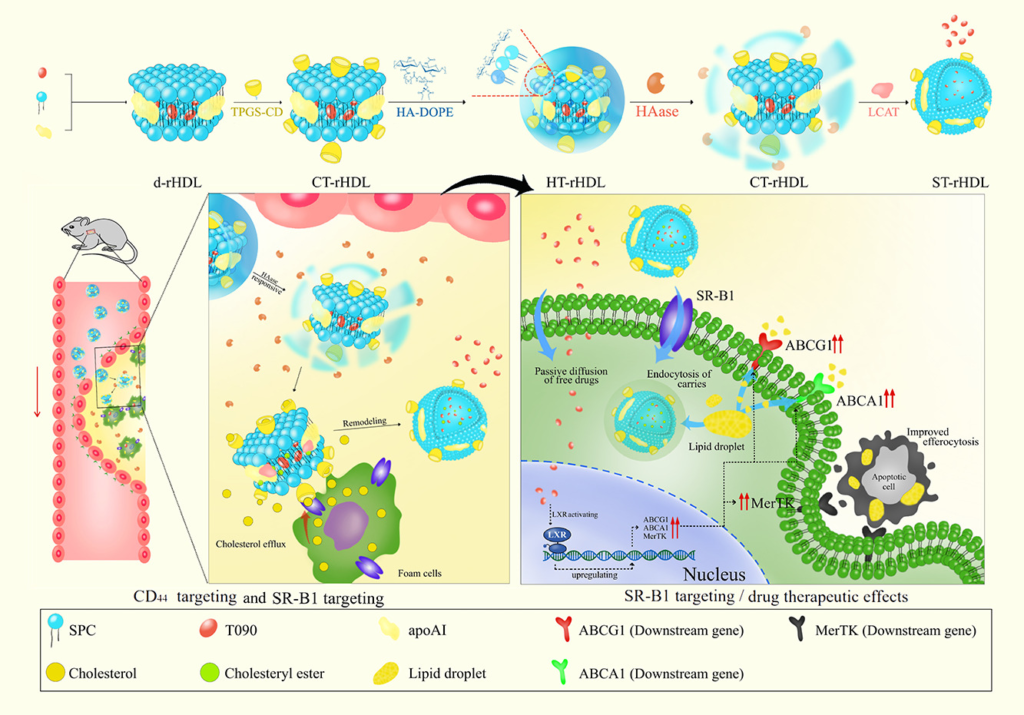
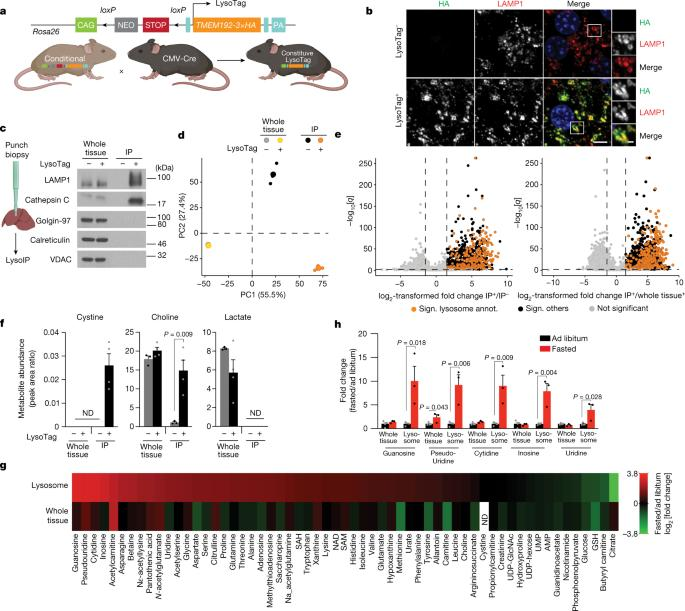
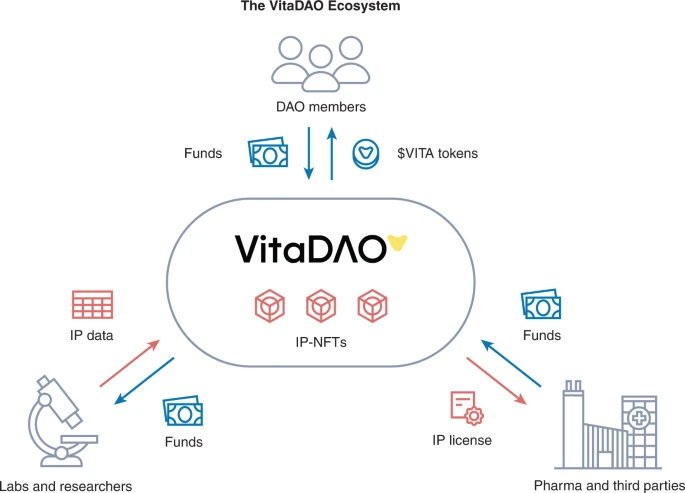
Cyclo Therapeutics Announces Publication of Phase 1 Data for Trappsol® Cyclo™ for the Treatment of Niemann-Pick Disease Type C1 (NPC1)
Cyclo Therapeutics, Inc. (Caroline Hastings, Benny Liu, Bryan Hurst, Gerald Cox and Sharon Hrynkow) Announces Publication of Phase 1 Data for Trappsol® Cyclo™ for the Treatment of Niemann-Pick Disease Type C1 (NPC1)
Artikle: https://www.sciencedirect.com/science/article/pii/S109671922200419X